Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
 O
O -pentadentate planar ligands designed for the strongest and selective capture of uranium from seawater
-pentadentate planar ligands designed for the strongest and selective capture of uranium from seawaterMizumachi, Takumi*; Sato, Minami*; Kaneko, Masashi; Takeyama, Tomoyuki*; Tsushima, Satoru*; Takao, Koichiro*
Inorganic Chemistry, 61(16), p.6175 - 6181, 2022/04
Times Cited Count:4 Percentile:58.46(Chemistry, Inorganic & Nuclear)Based on unique 5-fold equatorial coordination of UO
 , water-compatible pentadentate planar ligands, H
, water-compatible pentadentate planar ligands, H saldian and its derivatives, were designed as strong and selective capture of UO
saldian and its derivatives, were designed as strong and selective capture of UO
 in seawater. In the simulated seawater condition (0.5 M NaCl + 2.3 mM HCO
in seawater. In the simulated seawater condition (0.5 M NaCl + 2.3 mM HCO
 /CO
/CO
 , pH 8), saldian
, pH 8), saldian shows the strongest complexation with UO
shows the strongest complexation with UO
 to form UO
to form UO (saldian) (log
(saldian) (log
 = 28.05
= 28.05  0.07), which is more than 10 order of magnitude greater than amidoxime-based or -inspired ligand systems most commonly employed for U capture from seawater. Good selectivity for UO
0.07), which is more than 10 order of magnitude greater than amidoxime-based or -inspired ligand systems most commonly employed for U capture from seawater. Good selectivity for UO
 from other metal ions coexisting in seawater was also demonstrated.
from other metal ions coexisting in seawater was also demonstrated.
 ssbauer spectroscopic parameters
ssbauer spectroscopic parametersKaneko, Masashi
Hosha Kagaku, (35), p.36 - 39, 2017/03
This paper is an article for research introduction by winner of the Japan Society of Nuclear and Radiochemical Sciences Encouragement Price 2016. It was mentioned about the achievements which revealed the spin transition behavior of iron complex and the separation mechanism of actinides from lanthanides.
Ishimori, Kenichiro*; Watanabe, Masayuki; Kimura, Takaumi; Yaita, Tsuyoshi; Yamada, Takashi*; Kataoka, Yumiko*; Shinoda, Satoshi*; Tsukube, Hiroshi*
Chemistry Letters, 34(8), p.1112 - 1113, 2005/08
Times Cited Count:13 Percentile:44.68(Chemistry, Multidisciplinary)Stereochemical substitution on tripod ligand significantly offered efficient separation of trivalent actinides from trivalent lanthanides. Liquid-liquid extraction using chiral tris(2-pyridylmethyl)amine ligands as an extractant exhibited high efficiency and selectivity for trivalent actinides. A combination of chiral ligand and 2-bromodecanoic acid further enhanced extraction performance for trivalent actinides.
Kaneko, Masashi; Watanabe, Masayuki; Matsumura, Tatsuro
no journal, ,
In Japan Atomic Energy Agency, the extraction reagents for the inter- and intragroup separation of mainor-actinides from lanthanides have been developed in order to construct the partitioning and transmutation process. We aim to reveal the separation mechanism using various extraction materials at a molecular level and to design the novel extraction reagents. In this study, we apply density functional calculations to the separation behavior of Am(III) from Eu(III) by DGA and NTA ligands. All metal compounds were modeled by referring the literatures. As the result, DGA ligand selectively coordinates to Eu(III) than Am(III), on the other hand, NTA ligand preferably coordinates to Am(III) than Eu(III), being consistent with the experimental selectivity.
 ssbauer spectroscopic parameters
ssbauer spectroscopic parametersKaneko, Masashi
no journal, ,
This study aims to perform the optimization of density functional methods using experimental data of M ssbauer isomer shifts and apply these methods to d, f-block coordination chemistry. In the d-block complexes, the validity for the spin-crossover (SCO) switching behavior of iron(II) assembled complexes was confirmed. The application of this method indicated that whether SCO occurs or not depends on the dihedral angle between iron atom and pyridine plane. In the f-block complexes, the benchmarking of computational method was performed using
ssbauer isomer shifts and apply these methods to d, f-block coordination chemistry. In the d-block complexes, the validity for the spin-crossover (SCO) switching behavior of iron(II) assembled complexes was confirmed. The application of this method indicated that whether SCO occurs or not depends on the dihedral angle between iron atom and pyridine plane. In the f-block complexes, the benchmarking of computational method was performed using 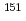 Eu and
Eu and  Np M
Np M ssbauer isomer shifts. The optimized method reproduced the separation behavior of Am(III) ion from Eu(III) ion and implied that the difference in the bonding contribution between 5f(Am) and 4f(Eu) attributes to the selectivity of Am(III) compared with Eu(III).
ssbauer isomer shifts. The optimized method reproduced the separation behavior of Am(III) ion from Eu(III) ion and implied that the difference in the bonding contribution between 5f(Am) and 4f(Eu) attributes to the selectivity of Am(III) compared with Eu(III).
Kaneko, Masashi; Watanabe, Masayuki; Matsumura, Tatsuro
no journal, ,
In Japan Atomic Energy Agency, the extraction reagents for the inter- and intragroup separation of mainor-actinides from lanthanides have been developed in order to construct the partitioning and transmutation process. We aim to reveal the separation mechanism using various extraction materials at a molecular level and to design the novel extraction reagents. In this study, we design a novel TPEN-type extraction reagent in which carbonyl donors are introduced and predict its separation performance of Am(III) from Eu(III) using density functional calculations. We modeled molecular complexes by referring single crystal structures and compared the stability between Eu(III) and Am(III) complexes. As a result, the relative stability of Am complex to Eu complex increased and its separation factor was improved by an order from five to twenty compared to the prediction value by non-substituent TPEN ligand.